Which Best Describes the Ph of Acid Deposition
Calculate the ratio of the. The lower a substances pH less than 7 the more acidic it is.

Acid Deposition Acid Rain What Is It Why Do We Care Ppt Download
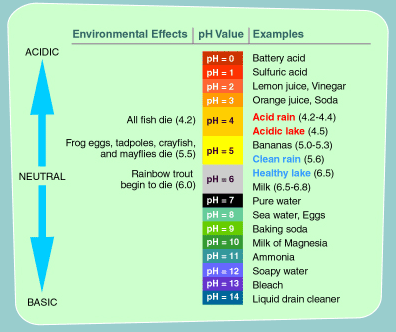
Acidic deposition or acid rain describes any form of precipitation including rain snow and fog with a pH of 55 or below Note.

. The land area that supplies water. Spokespeople for several local industries claim that the drop in pH is unlikely to have a negative effect on organisms living in the lake. Acid rain has a pH value of 30 whereas normal rain has a pH value of 56.
PH values below 7 are acidic. The soil samples are the same size and contain the same ratios of sand silt and clay. A more precise term is acid deposition which has two parts.
This gives rise to a pH of 7. Acidity and alkalinity are measured using a pH scale for which 70 is neutral. PH strong and weak acids and acid deposition.
Acid deposition has caused a lakes pH to drop below 50. Which of the following best describes a potential unintended negative consequence of implementing measures to control acid deposition. It is slightly acidic because carbon dioxide CO 2 dissolves into it forming weak carbonic acid.
The higher a substances pH greater than 7 the more alkaline it is. Topics 83 84 and 85 Exploring the relative strengths of acids and bases and how we calculate pH. Acid rain usually has a pH.
The result is a mild solution of sulfuric acid and nitric acid. Normally water has an equilibrium amount of hydrogen H and hydroxide ions OH- that are at a molar concentration of 10-7 moles per liter. Scientists are interested in how the severity of acid deposition affects the soil of the red spruce forests.
It often results when the acidity of normal precipitation is increased by sulfates and nitrates that are emitted into the atmosphere from burning fossil fuels. This form of airborne contamination is. Or biological processes is called- 1 point A Erosion B Weathering C Climate D Deposition 2.
The relative acidity of water is usually reported as the pH of the solution defined as the negative logarithm of the hydrogen ion concentration pH -log H. A A reduction in environmental lead leading to decreased negative health effects in humans and wildlife B A reduction in sulfur for crops as a result of less acidic rainfall leading to an increased need for. Which of the following statements describes the difference between physical and chemical weathering.
Sunlight increases the rate of most of the SO 2 and NO reactions. Vinegar has a pH of 3. Acid rain is a broad term used to describe several ways that acids fall out of the atmosphere.
They design a laboratory experiment in which rainwater of different pH values is used to water soil samples taken from red spruce forests. Normal rain has a pH of about 56. Rain and snow are already naturally acidic but are only considered problematic.
Acid deposition commonly known as acid rain occurs when emissions from the combustion of fossil fuels and other industrial processes undergo complex chemical reactions in the atmosphere and fall to the earth as wet deposition rain snow cloud fog or dry deposition dry particles gas.
Acid Rain Students Site Ph Scale

No comments for "Which Best Describes the Ph of Acid Deposition"
Post a Comment